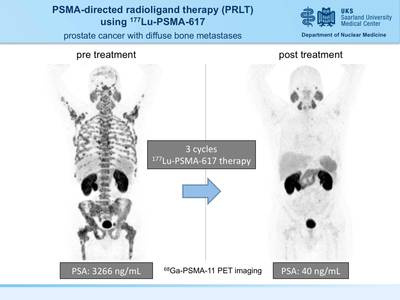
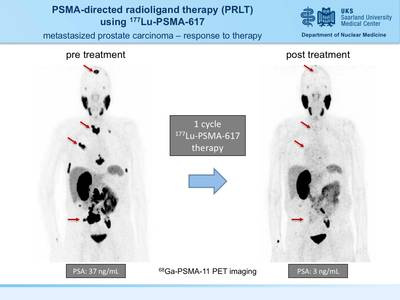
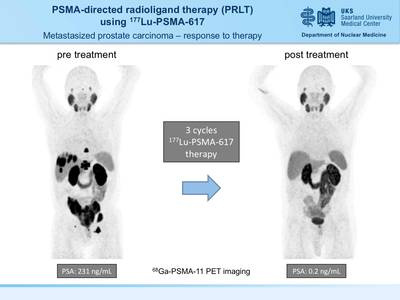
| PSMA-directed molecular imaging in prostate cancer PET / CT examination with 68 Ga-PSMA ligands
A new, highly sensitive marker for PET / CT has now been introduced at the Clinic for Nuclear Medicine at Saarland University Hospital in Homburg in order to identify tumor foci in patients with prostate cancer more precisely. The radiopharmaceutical gallium-68-PSMA ligand binds to prostate-specific membrane antigen (PSMA) on the surface of tumor cells and thus enables imaging with very high contrast. In the process, the smallest tumor settlements can be discovered that could escape conventional imaging.
How does the investigation work? A small amount of the gallium-68 labeled PSMA ligand ( 68 Ga-PSMA) is injected into a vein for examination. After the injection, the 68 Ga-PSMA will reach the diseased tissue via the blood vessel system and accumulate significantly more in the diseased cells compared to normal tissue. In order to observe and visualize this distribution in the body, the PSMA was labeled with a short-lived radioactive substance (Gallium-68) before the injection. Its physical half-life is only 68 minutes. The PSMA will increasingly accumulate the diseased tissue. With the help of the PET / CT scanner, the enrichment site can be localized by means of the radiation from the Gallium-68, and the tissue sought can be depicted graphically. In order to enable a reliable assignment to known structures (lymph nodes, soft tissues), a computed tomography is additionally carried out and the 68 Ga-PSMA PET images are superimposed with the CT images and evaluated together (PET / CT). Foci found in PET can thus be precisely assigned to the respective anatomical structures.
documents Please bring all relevant documents with you to the examination (in particular pictures and findings from previous examinations, also in digital form on CD and doctor's letters). For possible intravenous contrast medium administration as part of CT (computer tomography), please have your family doctor determine the creatinine value in the blood as soon as possible - ideally in the week before the examination. The result of the blood test should guide you.
examination procedure
The PET-CT device is open upwards (towards the head) and downwards (towards the legs) and cannot be compared to a tube. If there is pronounced “claustrophobia” (claustrophobia), an anxiety-relieving premedication may be necessary or advisable. Please keep in mind that you are not roadworthy with such an anxiolytic medication on the day of the examination! In the absence of persistent “claustrophobia” (99% of cases), no such medication is required and you can also drive yourself accordingly (before and after the examination).
Side effects: No side effects are expected from the injection of the radioactive tracer itself. Side effects can result from the administration of iodine-containing contrast medium in the context of any accompanying contrast medium-assisted CT (intolerance reaction, hyperthyroidism, aggravation of pre-existing renal insufficiency). If you have had a contrast medium reaction in the past (reddening of the skin, itching, swelling, shortness of breath, etc.), please be sure to point this out to our doctors during the patient's medical consultation.
Radiation exposure: The radiation exposure (radiation exposure) by the gallium PSMA is approximately in the range of twice the natural annual radiation dose (approx. 4-6 mSv). It is below the radiation dose of most conventional CT examinations. Acute and / or chronic radiation damage from gallium PSMA is not expected or has been observed.
Documents to bring Please bring your current documents with you to the examination, eg a CD / DVD with the image data of your current computed tomography or MR examination. Before each PET / CT examination, your family doctor should take a blood sample and determine the values for "creatinine" and "TSH". We kindly ask you to bring them to the examination.
Important: You do not have to be fasting on the day of the examination . You can have a light breakfast. The radiopharmaceutical is made for the patient and cannot be stored. It is imperative to keep your appointment or to inform us in good time if you do not show up .
| DURATION & PRICE |
You can send your findings to Dr Ezziddin via addresses in the link below, asking for comments or a second opinion:
Please enclose the following findings
|